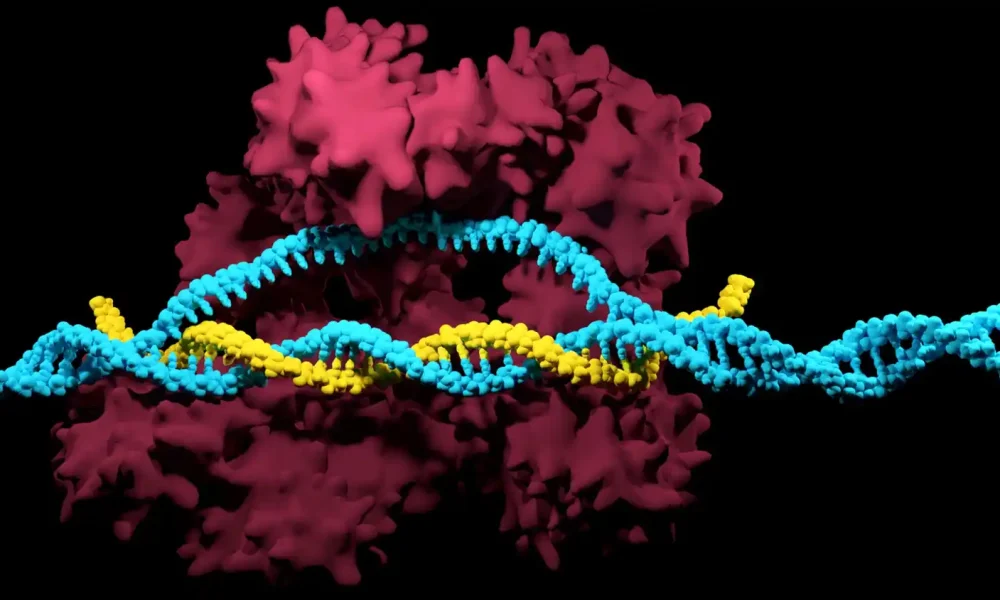
Expanding CRISPR’s Potential
Since its discovery, CRISPR (“Clustered Regularly Interspaced Short Palindromic Repeats”), which won the 2020 Nobel Prize in Chemistry, has revolutionized medicine and biotechnology.
Source: Nobel Prize
This is because CRISPR is the first method for gene editing that allows for very precise targeting of a specific genetic sequence, allowing for correcting genetic errors either in vitro or in vivo without risking unwanted mutations.
This is important as undirected gene insertion has been linked to major problems, notably cancer risks, making their therapeutic use difficult and controversial.
CRISPR can be used in multiple ways to interrupt a gene already present, delete a specific sequence, or edit/insert the right genetic sequence.

Source: CRISPR Therapeutics
This turned into a medical breakthrough with the FDA approval for the first CRISPR-based therapy in 2023, developed by CRISPR Therapeutics for genetic blood diseases (follow the link for a dedicated report about CRISPR Therapeutics).
However, CRISPR therapies and genetic engineering are only able to modify one gene at a time and can be difficult to design.
So, it could be a new era that is opening for CRISPR technology, thanks to the work of researchers at Yale University, who developed a new CRISPR method that can modify multiple genes at once.
It was a breakthrough study that brought together a massive team of researchers: no less than 37 persons are mentioned as authors of the study1 published in Nature Biomedical Engineering under the title “Cas12a-knock-in mice for multiplexed genome editing, disease modeling, and immune-cell engineering”.
It could help generate new disease and treatment models, such as genetic disease in the liver, lung cancer, and skin cancer, and accelerate further research in the field.
The Many CRISPR Systems
When CRISPR is discussed, either in its initial Nobel Prize-winning discovery or the already FDA-approved therapies, it is actually CRISPR-Cas9 that is discussed.
The “Cas” part of the system is the protein responsible for cutting the DNA strand and performing the gene editing itself, guided by a strand RNA. There are many other Cas proteins being studied, with probably many more to be discovered.
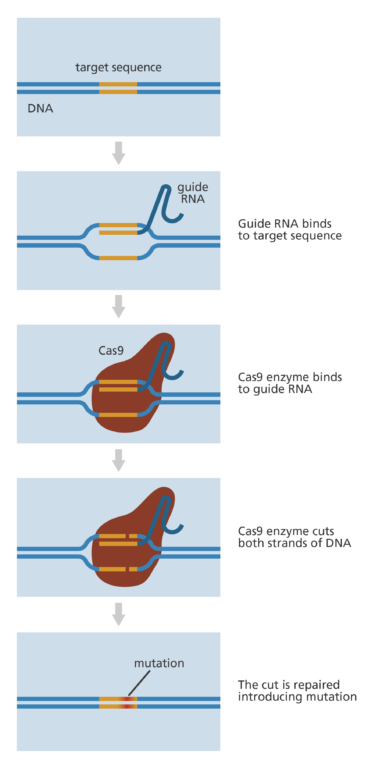
Source: YourGenome
It might even, in the long run, become commonplace for researchers to just design custom Cas systems, notably with “OpenCRISPR-1″, an open-source AI made to design a custom CRISPR system for specific situations. A computer simulation called CREME (Cis-Regulatory Element Model Explanations) might also help in understanding better how to optimize CRISPR systems.
Among these multiple CRISPR systems beyond CRISPR-Cas9, none has been as studied and is as close to practical application as CAs12a.
CRISPR-Cas12a
Since we first discussed it in 2023, much progress has been made on Cas12a technology.
CRISPR-Cas12a is a different system from Cas9 in a few aspects:
- Provides alternative cutting sites to what Cas9 can do.
- hard-to-solve problems with Cas9 could be workable with Cas12.
- It cuts DNA in a way that leaves a “sticky” section of DNA instead of the “blunt” cut of Cas9 and can be cut multiple times.
-
- This results in a higher chance of gene editing.
- There is no need for a transactivating crRNA (tracrRNA)for Cas12a, contrary to Cas9. Due to the smaller size, it would allow for easier multiplex genome editing.
- More than one gene can be modified at once with CAs12a.

Source: Wikipedia
It is this latest characteristic of Cas12a that has sparked the most interest for biologists, as many diseases or genetic engineering require multiple gene editing, insertion, and/or deletion.
So while it is theoretically feasible (although expensive) to perform a series of CRISPR-Cas9 gene editing when, for example, making a new variety of maize, it is not a viable option for multi-genic diseases.
Meanwhile, it is becoming clear that CRISPR has potential way beyond curing rare genetic diseases, with neurology, cancer (oncology), and metabolic issues the most likely to improve or save the lives of tens of millions of people.

Source: ARK Invest
The problem is that for most of the non-genetic disease-related therapies, managing multiple gene editing in vivo will be an absolute requirement.
Yale’s Cas12a Study
Diverse Targets
Before performing multi-gene editing on humans, we first need to have a better understanding of the effect of such an aggressive intervention on the genome.
The Yale researchers used lab mice to genetically engineer immune cells, including CD4+ and CD8+ T-cells, B-cells, and bonemarrow-derived dendritic cells. They also modified cancer-related genes and in-vivo liver tissues.
The CRISPR gene editing material was delivered to each target with retroviruses, adeno-associated viruses, and lipid nanoparticles, respectively.
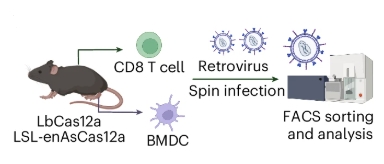
Source: Nature Biomedical Engineering
In order to expand the possibility of the method, they also used 2 variants of Cas12a.
Checking Efficiency
First, the researchers associated the mutation with a fluorescent protein to check if it was being performed properly.
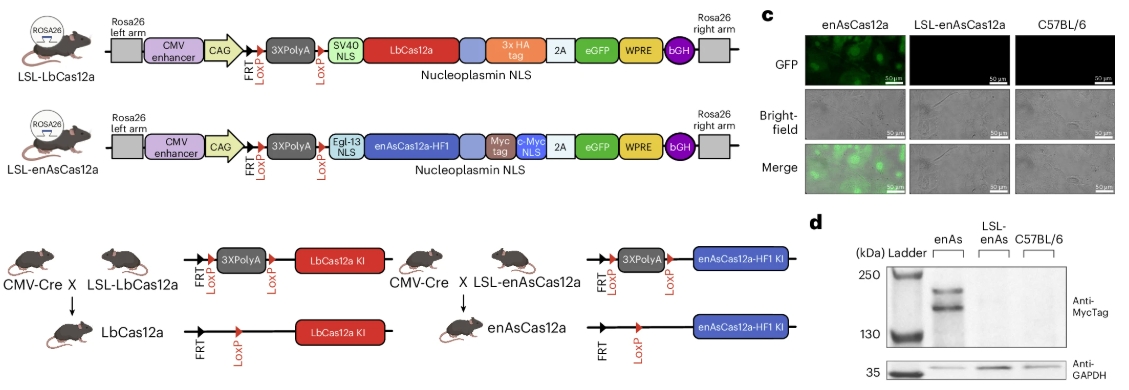
Source: Nature Biomedical Engineering
They then checked how the gene editing efficiency varied depending on the targeted organs. It appeared that the liver, brain, kidney, and lungs were among the easiest for achieving a high level of in-vivo gene editing.
This makes for very promising results, as these organs are the most important for the key medical targets of future CRISPR therapies.

Source: Nature Biomedical Engineering
Practical Potential
Once the concept had been proven to have potential, the researchers went on to test use cases of modifying immune cells and cancer cells.
When it came to immune cells, transformation efficiency, the metric measuring how much of the total exposed cells are genetically modified, was remarkably high, reaching up to 90-100%.
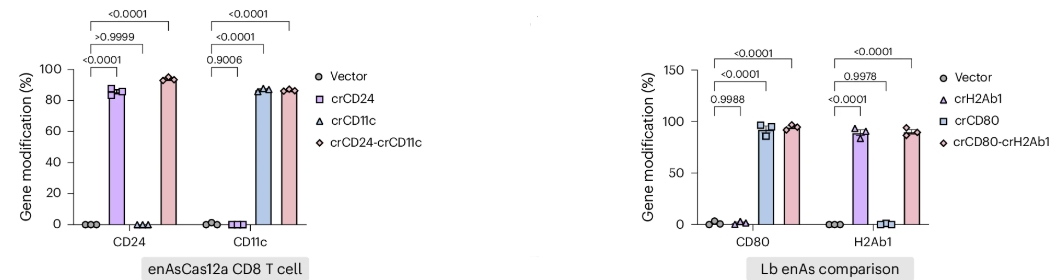
Source: Nature Biomedical Engineering
Another remarkable result, and an absolute requirement for any potential application of in-vivo therapies, is a very high rate of on-target mutation. This means that only the intended gene is being modified, and very few off-target gene editions occur.

Source: Nature Biomedical Engineering
DAKO
Pushing even further the possibility of CIRSPR-Cas12a technology, the researchers developed a novel dual-gene activation and knockout (DAKO).
This means that a single treatment can later on split and produce both the activation of one gene and suppression of another at once.
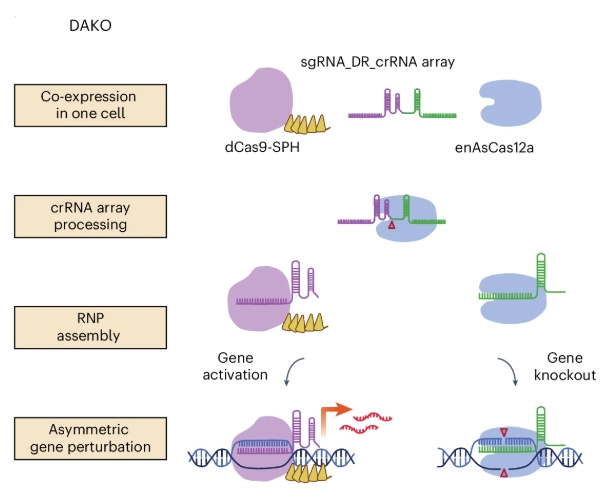
Source: Nature Biomedical Engineering
This is an important step for modifying cells with complex biochemical mechanisms, for example, one protein activating a mechanism and another one suppressing it. By performing DAKO gene editing, it becomes possible to both boost the desired activity beyond normal levels and remove the system that would inhibit this desired effect.
As unwanted or poorly managed retrocontrols are often a key reason for gene editing not performing as hoped, this DAKO system could prove to be a revolution, especially in oncology, neurology, and metabolic treatments.
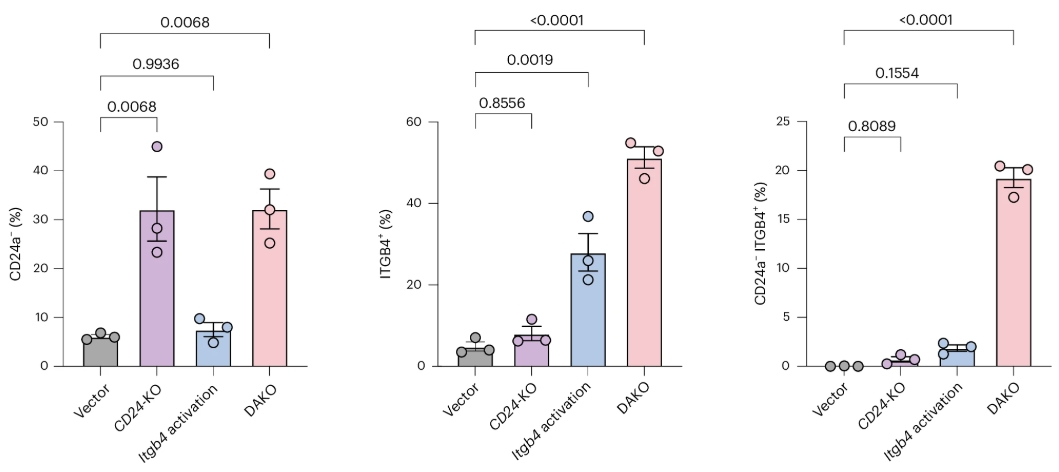
Source: Nature Biomedical Engineering
Animal Models
This new gene-editing system using Cas12a can be as precise as Cas9 but can perform multiple edits at once, and those edits can be both the activation and suppression of genes.
This makes it a perfect system to create a new biological model on which biologists can test their hypotheses.
“These mice can facilitate rapid and seamless workflows for in vivo therapeutic gene targeting, disease/tumor modeling, and primary immune-cell engineering.
Combined with pooled oligo library synthesizing and cloning, these mice could be used for high-throughput CRISPR screening to study complex genetic questions, such as effector and memory phenotype transition in immune cells.
Such animal models are very important to test potential treatment, or better understand complex diseases. So even if no therapy using Cas12a ever reaches the market, this alone would mean that this CRISPR variant will have a large impact on future biotech research.
Going Further
As it was proven in the study that the intensity of the gene editing can vary greatly depending on the organ, it is likely that further improvements can be achieved.
A key element will likely be testing new vectors for the gene editing system, either other viruses or other lipid nanocapsules. In particular, better vectors for the organs the least affected will be required for specific studies or diseases.
In the long run, it is most likely that gene editing therapies for complex diseases like cancer and neurological issues will use a system more akin to this cas12a system than the “traditional” mono-gene Cas9.
Cas12a Company
Editas
Editas Medicine, Inc. (EDIT -6.47%)
Editas was founded by CRISPR-Cas9 co-discoverer Jennifer Doudna. Editas started working with Cas9 but is now focused on a proprietary version of Cas12a that they engineered: AsCas12a.
You can read more about Cas12a’s unique properties in our dedicated article “What Is CRISPR-Cas12a2? & Why Does It Matter?”.

Source: Editas
You can also read an overview of all of Jennifer Doudna’s companies in the corresponding article “Top Jennifer Doudna Companies to Watch.”
Editas is focused on Sickle Cell Disease (SCD) and beta-thalassemia, 2 diseases where it lost the race for first treatment approval to competitors CRISPR Therapeutics and BlueBirdBio.
Overall, the SCD program (recently renamed reni-cell) has been delayed several times, sparking concern among investors, and has since been refocused on in-vivo therapy to distinguish it from already approved SCD therapies.
Nevertheless, Editas owns significant patents on CRISPR-Cas12, which has been used by researchers at the University of New South Wales, Australia, to develop a COVID-19 strip test, illustrating the technology potential beyond gene editing.
Editas also signed in 2023 a $50M deal with Vertex for the company to use Editas’ Cas9 IP.
Editas focuses on other CRISPR versions than the “classical” CRISPR-Cas9 and its research IP might come in handy in establishing partnerships and generating revenues without an FDA-approved product, on top of a cash runaway going into 2026.
As Cas12a seems to become increasingly proven as a best-in-class method for multi-gene editing, Editas’ expertise and pipeline focus on this CRISPR variant might prove a winning bet in the long run.
(You can read more about CRISPR companies in our corresponding article “Top 5 CRISPR Companies To Invest In”.)
Latest on Editas
Editas Medicine, Inc. (EDIT) Barclays 27th Annual Global Healthcare Conference (Transcript)
seekingalpha.com • March 11, 2025
Editas Medicine, Inc. (EDIT) Leerink Partners Global Healthcare Conference (Transcript)
seekingalpha.com • March 10, 2025
Editas Q4 Loss Wider Than Expected, Revenues Fall Y/Y, Stock Down
zacks.com • March 6, 2025
Editas Medicine (EDIT) Reports Q4 Loss, Misses Revenue Estimates
zacks.com • March 5, 2025
Editas Medicine, Inc. (EDIT) TD Cowen 45th Annual Health Care Conference Transcript
seekingalpha.com • March 3, 2025
Editas And Intellia: Strategic Shifts And Long-Term Investment Potential
seekingalpha.com • January 17, 2025
Studies Referenced:
1. Tang, K., Zhou, L., Tian, X. et al. Cas12a-knock-in mice for multiplexed genome editing, disease modelling and immune-cell engineering. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01371-2