Predicting Chemical Interactions
Drug discovery initially relied on randomly testing new chemical compounds or trying to find active compounds in nature and traditional medicine. A more modern version of this process is to find a target protein in the body and determine what molecule would attach to it (a “ligand”).
Typically, finding the right ligand involves a lengthy trial-and-error process. However, as computing power increases, the possibility of fully simulating proteins (made of tens of thousands, if not millions, of atoms) at the atomic level becomes realistic.
It is even more doable now that deep learning algorithms allow AI to “guess” the right answer instead of trying a “brute force” approach to these computing questions.
One such software is AlphaFold, a proprietary AI created by DeepMind, a branch of Alphabet/Google. With the release of AlphaFold 3 in May 2024, it is worth looking at the results from the previous version, AlphaFold 2, and if they are actually matching real-life protein-ligand interactions.
How Does AlphaFold Work?
AlphaFold is designed to learn from the known 3D configuration of 100,000 known proteins. From this, the machine started to find rules and guesses on how protein folds.
This is crucial information because proteins are effectively the machine that makes cells work. Their function depends not only on their sequences (which can be known from the genome) but also on their 3D configuration once the protein folds upon itself.
Source: News Medical
Technology like magnetic resonance and X-ray crystallography can be used to find this 3D structure, but this is a very expensive and slow process. Also, some proteins” 3D configurations cannot be found with these techniques.
Lastly, the way a protein interacts with other chemicals is key for most potential medicines to be effective. This adds another layer of complexity to the question.
With 300,000 different proteins in the human body and 300,000,000 different proteins on Earth, another method like AlphaFold is needed.
Back Testing AlphaFold 2
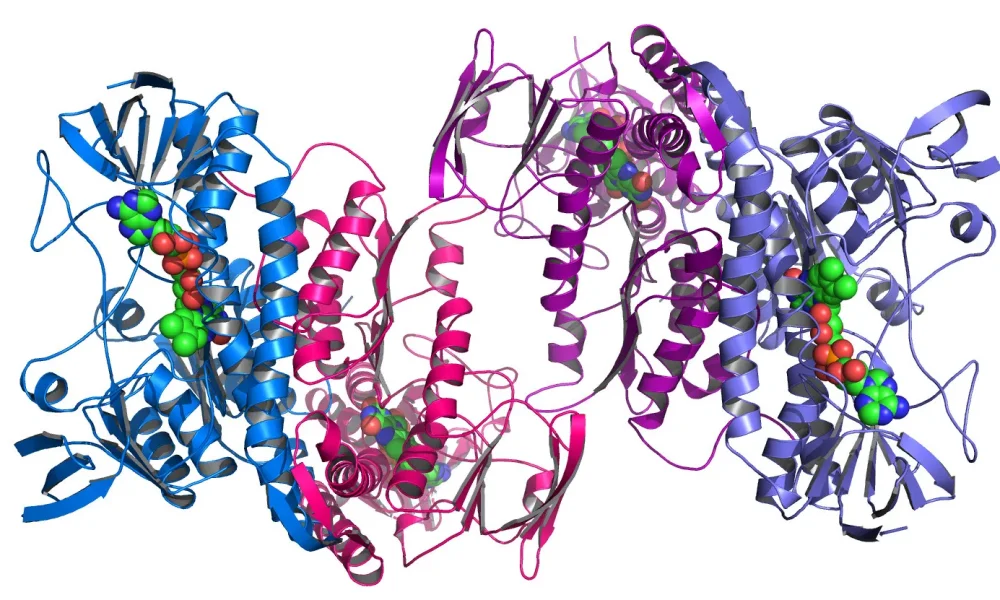
Checking AlphaFold 2’s accuracy is what researchers did in a scientific paper titled “AlphaFold2 structures guide prospective ligand discovery”.
The work was done through a massive collaboration between 20 researchers at the University of California, the University of North Carolina, Stanford University, Harvard Medical School, and Chemspace LLC in Ukraine.
The researchers used two proteins, sigma-2 and 5-HT2A, involved in neuropsychiatric diseases like Alzheimer’s disease and schizophrenia. These proteins were used because AlphaFold 2 had not been trained on any data involving these proteins, allowing it to double-check if it could “deduce” new findings from scratch.
Retrospective Study
The first thing the researchers did was ask AlphaFold 2 about the interactions with ligands they already knew bind to the protein in real life. In some cases, the interactions were predicted correctly, but not always.
This is not really news, and this limitation of retrospective studies for AlphaFold 2 has sometimes made researchers put into question its usefulness.
So, the next step was to check if AlphaFold could help find new protein-ligand interactions that were previously unknown.
Prospective Study
In this study, the researchers gave AlphaFold 2 the proteins’ structure and asked it to check for interaction with 1.6 million potential ligands/drugs.
AlphaFold predicted several drug candidates for each of the proteins.
The researchers then checked in real life if these potential ligands actually worked.
Researchers determined that the proportion of compounds that altered protein activity for each of the models was around 50% and 20% for the sigma-2 receptor and 5-HT2A receptors, respectively. A result greater than 5% is exceptional.
Considering that drug discovery is very much like trying to manually find a needle in a haystack, a 50% success rate of “guessing” where the needle is from the first try is indeed exceptional.
This could radically cut into drug discovery costs, as it allows to digitally comb through chemical databases containing millions of potential drug candidates.
The ideal situation is, of course, when the newly found drug candidate is already FDA-approved and has a known safety profile, which would speed up the clinical trials stage.
Out of the hundreds of millions of potential combinations, 54% of the drug-protein interactions using the sigma-2 AlphaFold2 protein models were successfully activated through a bound drug candidate. The experimental model for sigma-2 produced similar results with a success rate of 51%.
This shows that even if retrospective studies are not always conclusive, AI is an extremely powerful tool for new ligand drug discovery.
AI’s Entry Into Healthcare
Drug Discovery
Drug discovery is one big segment of the potential of AI in healthcare, especially as the cost to develop a new drug has grown over the past decades, often reaching a bill in the billion-dollar range or more.
AlphaFold is just one example, and assessing properly the safety of a drug, or finding new ways to produce it efficiently are also possible. This does not have to be with proprietary software either, with the appearance of open-source AI, including for gene editing as we discussed in “AI-Enabled Gene-Editing Made Possible with ‘OpenCRISPR-1’”.
Finally, AI drug discovery can target non-human proteins as well, for example finding from scratch new classes of antibiotics, as discussed in “MRSA is Increasingly Common in HealthCare Settings – Has AI Just Given a Tool to Fight Back?”.
AI Diagnosis and Patient Management
AI is now being used to analyze radiology images to determine the presence of cancer. It is even more accurate than human doctors when it comes to detecting ear infections, as discussed in our article “AI Poised to Become Invaluable Medical Diagnosis Tool”.
It can even determine when TXA, a risky but often life-saving drug, should be administrated after a trauma, as discussed in “Outdated Emergency Protocols Primed to be Modernized by Machine Learning”.
LLMs (Large Language Models) might also soon become good diagnosticians, helping patients either in collaboration with doctors or by themselves.
AI Data Management
Patient data used to be limited to a few radios or scanners, blood analyses, and notes from doctors.
Soon they will include lifestyle data (diet, exercise), wearable monitoring (sleep, heart rate, blood pressure, blood sugar), and even full genomic data.
AI can also help doctor productivity by prefilling patients’ files and helping with integrating multiple software and data sources.
Finding the relevant data and determining how to improve health outcomes from it will increasingly rely on bringing together these data, and having AIs analyzing them.
This will equally be true for population-scale datasets allowing us to find new ways to improve public health by finding previously unknown links between diseases and lifestyle or chemicals.
Robotics
Surgery is increasingly done with robots, allowing for a more precise treatment and comfort for the surgeons. And this sector is growing very quickly.

Source: Grand View Research
Robots can also allow surgeons to be hundreds or thousands of kilometers away, controlling the robot remotely. We looked into the leaders of this medical revolution in our article “Top 5 Robotic Surgery Stocks”.
Advanced Implants
One more futuristic, but not anymore limited to science fiction, use of technology in healthcare are artificial implants.
Exoskeletons are now entering the set of solutions to help patients, as illustrated in “Independence and Mobility through Robotics – How Exosuits Can Help Those with Parkinson’s Disease”.
A further step will be direct human-machine interfaces, notably through chips like Elon Musk’s Neuralink. It could be used to directly control artificial limbs or computers with just a thought.
Brain implants, exoskeletons, and prosthetics are just a few components of the revolution we explored in “Next-Level Evolution: Enhancing the Human Body With Anthrobots, Organic Transistors, Brain Implants, and More”.
AI Drug Discovery Companies
(Companies on this list are going to use AI tools, such as NVIDIA hardware. However, we are focusing here on purely drug-discovery companies and not AI in general or tech companies like Google).
1. Schrödinger, Inc.
The company specializes in physics-based models to find the best possible molecule for a given goal, balancing out conflicting metrics like potency, solubility, half-life, synthesizability, etc.
It also uses “normal” machine learning, but the addition of a physics-based model allows it to be tested in entirely novel fields for which no data set exists to “train” the AI. This allows Schrödinger to go from 1 billion potential molecules to just 8 solid candidates in a matter of days, exclusively through digital calculation.

Source: Schrodinger
Schrödinger signed with Bayer a 5-year collaboration agreement in 2020 for revenue of $10M. The idea of the agreement is to use Schrödinger technology together with Bayer in-silico prediction models.
Another recent partnership is with Lilly in 2022, with up to $425M in total milestone payments for successful discovery.
Past collaborations included Takeda, Sanofi Bristol Myers Squibb, and other smaller pharmaceutical companies.
Overall, Schrödinger is building a growing portfolio, including more and more proprietary and fully-owned molecules. It currently has 8 products in its proprietary pipeline, with 2 in phase I of clinical trials. And 23 products in partnered programs and collaborations, with 5 in phase I and 3 in phase II of clinical trials.

Source: Schrodinger
While not pre-revenue, the company is still not profitable, focusing on expansion and R&D spending to improve its technology. It should not be a serious concern in the short term, as the company has several years of operation worth of cash on its balance sheet.
It is also looking at expanding toward new segments beyond drug discovery, like complex biopharmaceuticals or even materials like chemicals, batteries, or polymers.
Source: Schrodinger
Investors will want to keep an eye on the new collaborations, as they will reflect the advances of Schrödinger’s technology, as assessed by the leaders in the industry from confidential data, as well as possible success in expanding the core technology to new markets.
2. Exscientia
The company is using AI to develop precision therapies. It runs a “full stack” AI drug discovery technology with dedicated software at every stage of the drug discovery process.

Source: Exscientia
Instead of looking at existing molecules, Exscientia’s Precision Design AI designs custom molecules to match the target found by its Precision Target AI.
Exscientia’s technology reduces 70% of the time required to go from a biological target to finding a corresponding drug and an 80% more capital-efficient process.
Part of the time and cost saving comes from a highly automatized process, with “comprehensive robotic automation across the entire experimentation cycle”.
This resulted in 4 compounds in early clinical stages, focusing mainly on oncology (cancer) and inflammatory diseases. The company shows $4B in pre-commercial milestones potential.

Source: Exscientia
The company is only starting to register revenues but has a long cash runaway worth several years of spending on its balance sheet.
By sitting at the junction between AI-drug discovery and precision therapy, Exscientia is aiming for two of the most transformative fields of medical science. Judging from the established partnership with Merck, Sanofi, and BMI, other established pharmaceutical companies also consider that the platform has great potential.