The Ultimate Battery
As batteries have become the basis of EV powertrains, their performance and safety profile have improved. So far, this has been achieved with variations of lithium batteries, either lithium-ion (lithium-nickel-manganese NMC & lithium-nickel-cobalt-aluminum NCA) or lithium-ferrum-phosphate (LFP) batteries. It was a transformative technology that rightfully earned its inventors the 2019 Nobel Prize in Chemistry (follow the link for the history of lithium-ion invention).
Until now, these batteries were expected to keep dominating the battery market, thanks to their extremely high energy density.
Source: S&P Global
There is, however, a limit to how much energy classical lithium-ion batteries can hold. This is why researchers are looking at other options, of which one of the most likely to bear fruit is solid-state batteries.
Solid-state batteries are expected to be safer, more energy-dense, and more durable than traditional lithium-ion batteries. However, they are very hard to manufacture reliably at scale in a cost-efficient manner, which has slowed down their adoption.
This could change, and new insights about what makes solid-state batteries fail are coming from researchers working at Princeton University, Purdue University, the University of Michigan, and Brookhaven National Laboratory.
They published their most recent discoveries in two scientific papers in Advanced Energy Materials1 and ACS Energy Letters2, respectively, under the titles “Lithium Kinetics in Ag–C Porous Interlayer in Reservoir-Free Solid-State Batteries” & “Filament-Induced Failure in Lithium-Reservoir-Free Solid-State Batteries”.
They also analyzed the current state of the art of battery science regarding anode-free batteries and published it in Nature Materials3, under the title “Electro-chemo-mechanics of anode-free solid-state batteries”.
Anode-Free Solid State Battery
The idea of solid-state batteries is to replace the liquid electrolyte in lithium-ion with a layer of solid metal. This is the major source of gain of efficiency, as electrolytes are heavy and voluminous.

Source: University Of Chicago
This also improves the safety profile, as electrolyte solvents are usually flammable, creating rare but spectacular battery fires that have been giving a bad reputation to early EVs.
Another step has been explored by researchers recently, removing entirely half of the battery. Batteries are made of a cathode and an anode, each with a different electric charge.
Anode-free batteries completely forgo the need for an anode,
“Instead, ions flow from the positive cathode directly to the current collector at the opposite end of the battery. The ions then plate onto the current collector itself, forming a thin metal layer as the battery charges.”

Source: Princeton University
In their analysis of anode-free technology today, the Princeton researchers argue that the main issue to progress the technology further is a poor understanding of the mechanical effect of the charging-discharging cycle, more than chemical reactions.
The mechanisms governing charge-discharge cycling of anode-free batteries are largely controlled by electro-chemo-mechanical phenomena at solid–solid interfaces, and there are important mechanistic differences when compared with conventional lithium-excess batteries.
Solid-State Challenges
In a classical battery, the connection to the electrodes (anode & cathode) is relatively easy, as the electrolyte is in liquid form. In a solid-state battery, the solid metal needs to perfectly stay in contact with the current collector.
If this is not perfectly even, areas with good contact become hotspots, while areas with poor contact form voids.
To understand why this happens, researchers need to have a perfect understanding of the complex process happening during the battery charge and discharge. This is not only a chemical phenomenon, but also a mechanical one, with the material changing shape slightly over time.
In the first paper, they discovered that pressure can play an important role in how the solid-state metal reacts.
Low-Pressure Issues
Scanning electron microscopy reveals how lithium has increasing surface contact as pressure increases. So it means that too low pressure does not do enough to improve the uneven contact caused by those surface irregularities.

Source: ACS Publication
Ultimately, the uneven plating led to the formation of sharp metal filaments that, like tiny needles, could pierce the solid electrolyte and cause the battery to short-circuit.
High-Pressure Issues
While the high pressure can create uniform plating and stripping, it is not a magical solution.
The researchers found that it forced the electrolyte and the current collector together so intensely that any imperfections on either were magnified until the mechanical stress caused fractures to form.

Source: ACS Publication
Using X-ray tomography, the researchers managed to map these cracks forming under high pressure.
As stack pressure is increased from 2 to 10 MPa, the entire volume of cracking grows. Many cracks extend to the counter electrode side (Figures 3b–e and S10), and a single lithium dendrite reaching the counter electrode can cause a short circuit.

Source: ACS Publication
Overall, finding the sweet spot of low enough pressure, but efficient contact will be the end goal for the battery industry.
“The Holy Grail in this area will be to figure out how to maintain solid contact at low pressures since manufacturing a defect-free electrolyte is practically impossible. If we want to realize the potential of these batteries, we have to solve the contact issue.”
Pr. Kelsey Hatzell – Associate professor of mechanical and aerospace engineering
Better Plating
Achieving more uniform plating is the topic of the second paper published by Pr Hatzell’s team and their collaborators in other universities and laboratories.
They found that a thin layer of coating between the current collector and the electrolyte facilitates better ion transport. They tested multiple designs for this coating.
Ultimately, they found that the best option was interlayers made from carbon and silver nanoparticles. The silver in these interlayers formed alloys with ions during battery charge and discharge, enabling even plating and stripping from the current collector.
However, the details of how the silver particles are made matter a lot. When using larger nanoparticles of 200nm (nanometers), they formed spindly, uneven metal structures on the current collector. This reduced capacity and eventual battery failure over several charging cycles.
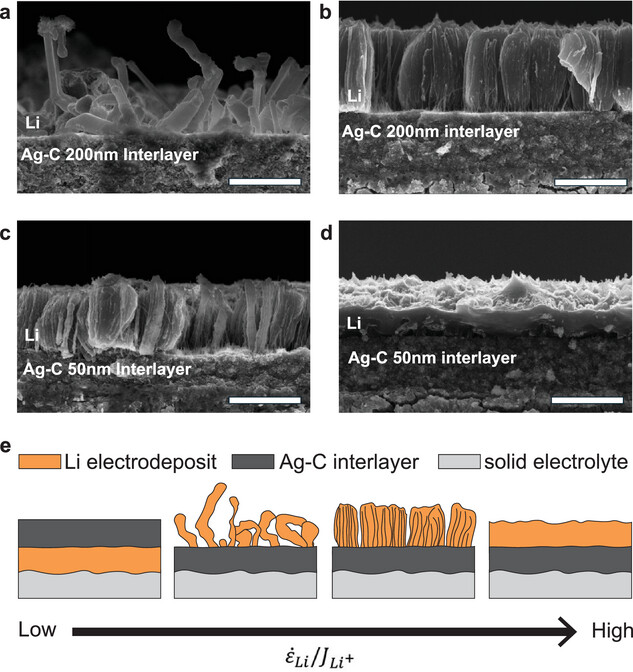
Source: Advanced Energy Materials
“Only a few groups have investigated the actual processes that occur in these interlayers. Among other findings, we demonstrated that the stability of these systems is linked to the morphology of the metal as it plates and strips from the current collector.”
Se Hwan Park – Postdoctoral researcher at Princeton University
50nm silver particles performed a lot better, creating denser and more uniform structures, leading to batteries with greater stability and higher power output.
“These findings can inform the strategy for fabricating these interlayers.
By reducing the size of the silver particles, we can make sure that we only get the advantages of the silver in the interlayer, which, in turn, could allow us to achieve good contact and uniform plating even at low pressures.”
Se Hwan Park – Postdoctoral researcher at Princeton University
Building Better Solid-State Batteries
For a long time, the solid-sate battery concept struggled to exit the lab and make it to the factory floor, with production at scale.
This is now changing, with countries like China, Japan, and South Korea having near-term plans to bring solid-state batteries to market.
For example:
“The challenge will be getting from research to the real world in only a few years. Hopefully, the work we’re doing now at MUSIC (Mechano-Chemical Understanding of Solid Ion Conductors) can underpin the development and deployment of these next-generation batteries at a meaningfully large scale.”
Pr. Kelsey Hatzell – Associate professor of mechanical and aerospace engineering]
Investing In Advanced Battery Technologies
Batteries are at the center of the trend of electrification, itself a major multi-trillion-dollar endeavor looking to remove fossil fuels from our power sources.
You can invest in battery-related companies through many brokers, and you can find here, on securities.io, our recommendations for the best brokers in the USA, Canada, Australia, the UK, as well as many other countries.
If you are not interested in picking specific battery companies, you can also look into battery ETFs like Amplify Lithium & Battery Technology ETF (BATT), Global X’s Lithium & Battery Tech ETF (LIT), or the WisdomTree Battery Solutions UCITS ETF, which will provide a more diversified exposure to capitalize on the growing battery industry.
Solid-State Battery Copany
QuantumScape
QuantumScape Corporation (QS -2.68%)
Since its foundation in 2010, Californian Quantum Scape has been a prominent startup in the solid-state battery space, remarkable by its move into the field early, and its independence from larger battery manufacturers also pursuing solid-state technology, like CATL (300750.SZ), Samsung, or LG Energy Solution (373220.KS).

Source: QuantumScape
One unique feature of QuantumScape batteries, which at the time was considered revolutionary, is that is use an anode-free design. It allows for ~15-minute fast charge (10-80% at 45 ºC) and the separator is nonflammable and noncombustible.

Source: QuantumScape
This also puts QuantumScape batteries in a league of their own when it comes to energy density and charging speed, massively outperforming leaders like Tesla (both its own design and CATL-made ones).

Source: QuantumScape
However, these remarkable performances have been regularly hindered by a struggle to ramp up production. It also forced the company to burn through its cash pile, leading to previous investors dilution and share prices decline.
This seems to be changing, since the 2024 agreement with PowerCo, the Volkswagen Group battery division, for a licensing deal for the design and mass production of QuantumScape batteries by PowerCo.
Under the non-exclusive licensing deal, PowerCo can manufacture up to 40 gigawatt-hours per year of electric vehicle batteries, with the option to expand to 80 GWh a year.
The sudden scaling-up of QuantumScape production seems to be linked to Cobra, the company’s next-generation solid-state battery separator equipment, a breakthrough in ceramics manufacturing.
Overall, Cobra should be integrated into production in 2025 and the first finished EV using QuantumScape batteries should be produced in 2026.

Source: QuantumScape
This could be a turning point for the company, moving 16 years after founding from a promising startup with interesting IP to generating growing revenues from a partnership with one of the largest automakers in the world.
In the meantime, investors should still expect some volatility in the stock price, but with a light at the end of the product development tunnel.
Latest on QuantumScape
Study Reference:
1. Se Hwan Park, et al. (2025) Filament-Induced Failure in Lithium-Reservoir-Free Solid-State Batteries. ACS Energy Letters. February 22, 2025 https://pubs.acs.org/doi/full/10.1021/acsenergylett.5c00004
2. Se Hwan Park, et al. (2024). Lithium Kinetics in Ag–C Porous Interlayer in Reservoir-Free Solid-State Batteries. Advanced Energy Material. 19 December 2024 https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/aenm.202405129
3. Stephanie Elizabeth Sandoval, et al. (2025). Electro-chemo-mechanics of anode-free solid-state batteries. Nature Materials. 02 January 2025 https://www.nature.com/articles/s41563-024-02055-z
