Finding The Right Hydrogen Catalyst
Hydrogen could, in theory, be a perfect fuel to store energy and power applications that are hard to electrify. This is because it presents a few almost ideal characteristics:
- The byproduct of its combustion is only water
- And the same can be said when it is used to produce electricity in fuel cells.
- It can burn at very hot temperatures, making it a good alternative to natural gas in metallurgy, chemical processes, etc.
- It only requires water as a resource for its production.
- Hydrogen is itself non-toxic and non-polluting.
However, the rise of a hydrogen-based economy has been hampered by the difficulty of producing hydrogen in a cost-effective manner. This is due to the fact that most green hydrogen (produced from green energy) is made through electrolysis, a process for now mostly based on expensive catalysts like platinum, ruthenium, or iridium, each very rare and expensive metals.
So as long as no better production method for hydrogen exists, it is unlikely that we will manage to see it replacing fossil fuel at scale.
Luckily, this is quickly changing. We previously covered a few of these possibilities, notably turning plastic waste into hydrogen, using nanorods of nickel as an alternative catalyst, or using titanium and nickel scrap metals (swarf) produced during the manufacturing of metal parts. Some newer options are now being added by researchers.
The first is the creation of self-optimizing catalysts1 by researchers at the Johannes Gutenberg Universitat (Germany) and Technical University of Darmstadt (Germany), Max Planck Institute for Polymer Research (Germany), Harbin Institute of Technology (China), and Shandong University (China). It was published in Angewandte Chemie under the title “Self-optimizing Cobalt Tungsten Oxide Electrocatalysts toward Enhanced Oxygen Evolution in Alkaline Media.”
The second one is the invention of a method to turn sewage sludge into green hydrogen and animal feed2 by researchers at the Nanyang Technological University (Singapore), Monash University (Australia), and University of Hong Kong (China). It was published in Nature Water under the title “Solar-driven sewage sludge electroreforming coupled with biological funnelling to cogenerate green food and hydrogen”.
Fixing Hydrogen Catalysts
A recurring issue with all hydrogen-producing catalysts is that they degrade over time. This can be due to a deposit forming on the reactive metals, or the metal layer itself slowly degrading and losing components at each catalysis cycle.
This is especially problematic for expensive catalysts of the platinum metals group, but it is also an issue for other types of metal-based catalysts.
So, it is important that the German and Chinese researchers of the first study discussed here have observed self-optimizing behavior with their new catalysts.
“What’s so unique about our catalyst is that it actually enhances its performance over time, while conventional catalysts either maintain their performance at a consistent rate or even lose some of their performance because they are insufficiently durable,”
Dr. Dandan Gao – Research Lead at Johannes Gutenberg University Mainz
Cobalt-Tungsten Catalysis
Solving Hydrogen Production Chokepoint
The researchers focused on so-called 3d to 5d transition metal oxides, formulated as mixed metal.
These are able to perform a chemical reaction called oxygen evolution reaction (OER), which is half of the reaction occurring during electrolysis of water into hydrogen, in both of the most common electrolyzer designs (AEM and PEM electrolyzers).
Source: SpectroInlet
“There are two reactions during the splitting of water. The hydrogen evolution reaction (HER), which produces hydrogen gas, and the oxygen evolution reaction (OER), which produces oxygen gas. The OER represents the bottleneck for the whole reaction. That’s why we are so committed to developing a catalyst that can promote the OER half-reaction.”
Dr. Dandan Gao – Research Lead at Johannes Gutenberg University Mainz
However, these novel potential catalysts are still poorly understood, with little knowledge of what exactly happens at the atomic level during the reaction, or even the electrochemical form the metal takes.
This lack of understanding is a big hindrance to developing a commercially viable solution, as it also limits the ability to anchor the catalysts to a stable substrate.
One-Step Deposit Method
The researchers used a copper oxide (L−CuO) microflower substrate with a diameter of 3–5 μm, previously developed in their lab in 2020.
They then used a chemical deposit method to create a layer of cobalt-tungsten alloy at the surface of the copper substrate.
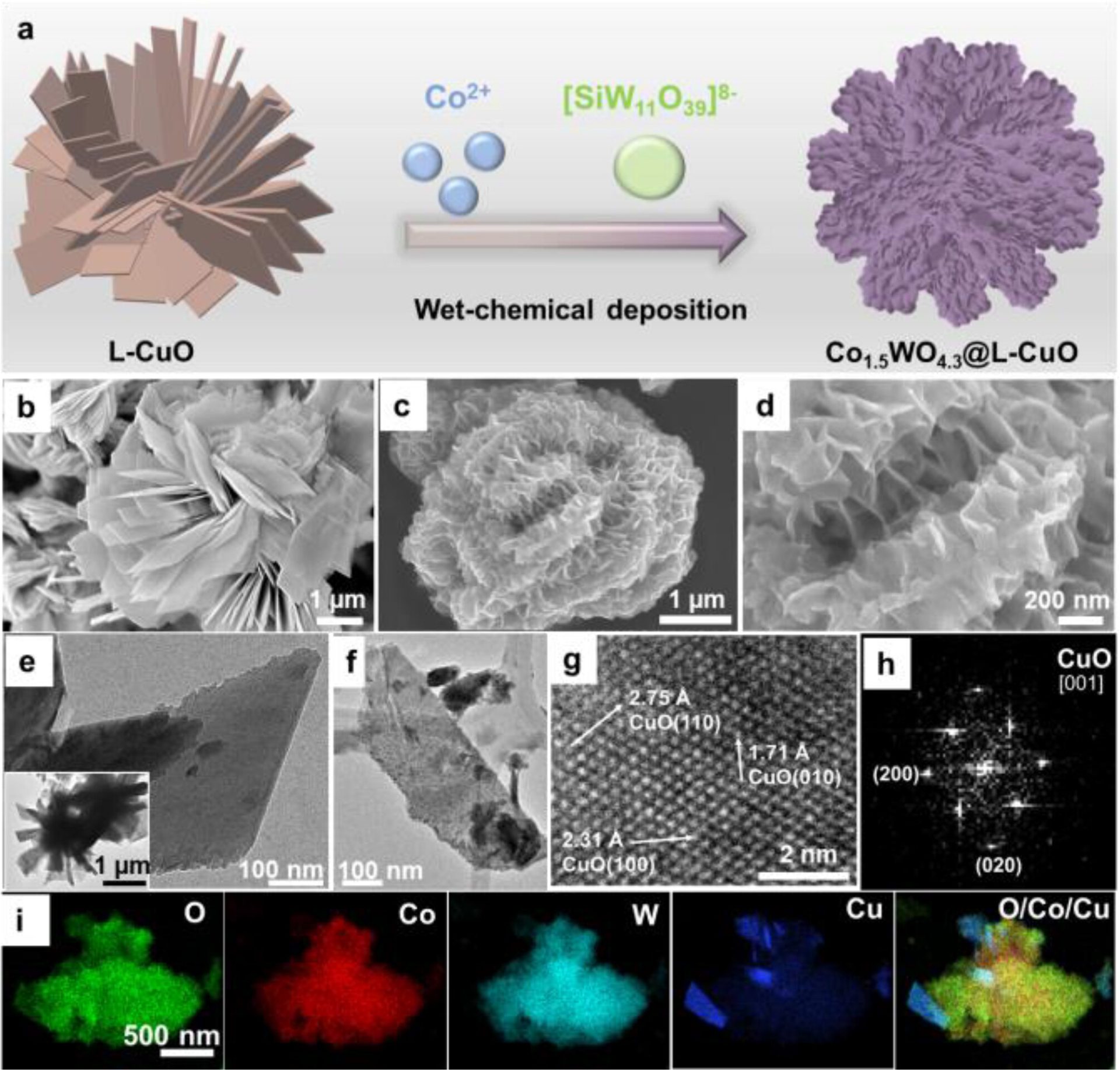
Source: Angewandte Chemie
Subsequent analyses revealed the complex microscopic structures of the material, using X-ray photoelectron spectroscopy (XPS), attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy, and Raman spectroscopy measurements.
It also confirmed that the catalyst is very firmly tied to the copper substrate.
“The development of viable and scalable deposition approaches is of utmost technological, economic, and ecological significance, enabling stable anchoring of OER pre-catalysts on selected promising substrates with high mechanical integrity”
Source: Angewandte Chemie
Self-Optimizing Catalyst
From these extremely detailed observations, the scientists discovered that the cobalt ions switch from the Co2+ form to the Co3+. At the same time, the tungsten ions also move from the W5+ to the W6+ forms.
As a result, over time, the electrochemically active part of the catalyst is transferred from the tungsten active site to the cobalt active site.

Source: Angewandte Chemie
The catalyst also changes in terms of surface hydrophilicity, or its ability to attract water (the fuel for hydrogen generation): over time, it becomes more hydrophile.
“In general, we recorded notably reduced overpotentials and increased current densities accompanied by a substantial increase in OER kinetics. All this is positive news for the hydrogen production of the future.”
Dr. Dandan Gao – Research Lead at Johannes Gutenberg University Mainz
This should be a powerful step into making transition metal oxides viable catalysts for hydrogen production.
Not only does this demonstrate that the team of scientists has developed a viable copper substrate for the catalyst, but also that such catalysts can be hyper-stable, and even improve over time.
It also provides the theoretical framework to evaluate the potential of other transition metal combinations not yet as well understood as cobalt-tungsten now is.
No Need For Catalysis?
Dealing with Cities’ Sludge
Meanwhile, hydrogen production could also start to come from the massive waste streams that our cities produce. This is at least the concept explored by Singapore, Chinese, and Australian researchers.
They focused on sewage sludge, a toxic byproduct of cleaning wastewater. These sludges are notoriously difficult to process and dispose of due to their complex structure, composition, and contaminants such as heavy metals and pathogens.
More than 100 million tonnes of sewage sludge are generated globally each year. Common disposal methods – such as incineration or landfill – are time-consuming, energy-inefficient, and contribute to environmental pollution.
Instead, it could become a source of animal feed and hydrogen at the same time.
Turning Sludge Into Resources
The researchers developed a 3-step process to treat the sludges.
First, they mechanically break down the sludges into a liquid. Then they remove the heavy metals from the organic material through a chemical treatment.
Next, they use an electrochemical process to transform the organic materials into valuable products: acetic acid and hydrogen gas, using specialized electrodes.
Finally, they feed a culture of bacteria able to harness light (cyanobacteria) to turn the leftover organic content into single-cell proteins suitable for animal feed.
Both the second and third steps are either directly (in the case of the bacteria) or indirectly with solar panels (in the case of the electrochemical treatment) powered by sunlight.
This makes the entire sludge recycling process entirely carbon-free, and actually carbon negative as it avoids carbon emissions from the sludge’s normal processing, and replaces other fossil-fuel-based sources for acetic acid, hydrogen, and animal feed.
Hydrogen can be used as a source of clean energy, and acetic acid is a key ingredient for food and pharmaceutical industries.

Source: Nature Water
High Efficiency
This method has been demonstrated to recover 91.4% of the organic carbon in sewage sludge and convert 63% of the organic carbon into single-cell protein.
This is much higher than traditional anaerobic digestion, which typically recovers and converts around 50% of organic materials in sewage sludge.
Overall, this reduces carbon emissions by 99.5% and energy use by 99.3% compared to traditional methods.
“We hope that our proposed method shows the viability of managing waste sustainably and shift how sewage sludge is perceived — from waste to a valuable resource that supports clean energy and sustainable food production.”
Dr Zhao Hu, Research fellow at Nanyang Technological University
In addition to this high efficiency, it also purifies the sludge from heavy metals, which tend to pollute landfills in the usual methods to deal with it.
This new method indicates that a revolution in how wastewater is dealt with in the world is possible, removing heavy metals (maybe for later recycling?) and producing useful acetic acid, hydrogen, and animal feed all at once.
Conclusion
A hydrogen economy will likely be one with a complex mesh of various hydrogen sources.
Most likely, one will be advanced catalysts performing water electrolysis into hydrogen, without requiring expensive rare metals. It would bring the cost of hydrogen low enough to make it an economically viable competitor to fossil fuels and other green alternatives.
Another likely source is to utilize better the massive millions of tons of waste products created by farming, wastewater, and other human activities. As these need processing anyway, it is by far better that we start processing them in such a way that pollutants (like heavy metals) are removed and new useful products are created.
And if this is done by being solely solar-powered, the better.
Tungsten Catalysis Company
Tungsten is emerging progressively as not only a super resistant metal, used in heavy industry and the defense sector, but also as a power catalyst useful for the chemical industry and to generate hydrogen.
It might even become a powerful high-temperature superconductor when woven in the right molecular configuration.
You can read a technical and investing overview about this resource in “Tungsten – The Secret High-Tech Metal”.
It is also a metal whose supply chain is almost entirely controlled by China, with one exception, Almonty Industries.
Almonty Industries
Almonty Industries Inc. (AII.TO +35.22%)
Almonty is a tungsten miner that is currently mostly producing from a mine in Portugal, in operation for the last 125 years.
The company has been working on expanding the Portuguese mine and owns undeveloped deposits in Spain.

Source: Almonty
The company’s most important project is the ongoing development of a new mine in Sangdong, South Korea. The mine contains more inferred resources than all of its other deposits combined.

Source: Almonty
As one of the only active and producing tungsten miners in Western countries, Almonty is a key strategic supplier for the defense industry. So, it is an important company for reducing dependence on Chinese supply.
The Sangdong mine’s location makes it a perfect supplier to the defense industry, with South Kore,a new giant in mass production of “low techs” military gear like tanks, artillery, and ammunition (compared to less tungsten-demanding fighter jets, aircraft carriers, etc.).
While China prepares to open a huge tungsten mine in Kazakhstan, Almonty is poised to “substantially shift the politics involved with securing tungsten” when the Almonty Korea Tungsten Project’s Sangdong mine comes online within a few months. When it begins production, it will be one of the world’s largest tungsten mines, accounting for 30% of the non-Chinese supply.
Lewis Black, director, president, and CEO of Almonty Industries
Almonty should start producing tungsten from the Korean mine in early to mid-2025.
Because of its strategic position as essentially the sole large supplier in the West, Almonty was offered a guaranteed price by Plansee. Plansee is a high-performance metal manufacturer and one of Almonty’s larger clients, as well as the owner of 15% of the company.
The minimum guaranteed price was $235/MTU (metric ton unit), with no upper threshold. As Sangdong Mine is aiming for cash costs of $110/mtu, this should virtually ensure a high-profit margin for the project.
With a lucky, almost perfect timing between the upcoming opening of Sangdong and a new trade war between Trump’s America and China, the stock price has reacted strongly and rose by 40% in just 2 days following the announcement of tungsten export restriction from China.
As tungsten becomes more and more important for high-tech applications, as well as geopolitical tensions, stay high, secure, non-Chinese tungsten supply is likely to come to generate a stable premium, with Almonty one of the strongest beneficiaries.
Study Reference:
1. Christean Nickel et al. (2025) Self-optimizing Cobalt Tungsten Oxide Electrocatalysts toward Enhanced Oxygen Evolution in Alkaline Media. Angewandte Chemie. 05 February 2025 https://doi.org/10.1002/anie.202424074
2. Hu Zhao, et al. (2024) Solar-driven sewage sludge electroreforming coupled with biological funnelling to cogenerate green food and hydrogen. Nature Water. Volume 2, pages1102–1115. https://doi.org/10.1038/s44221-024-00329-z